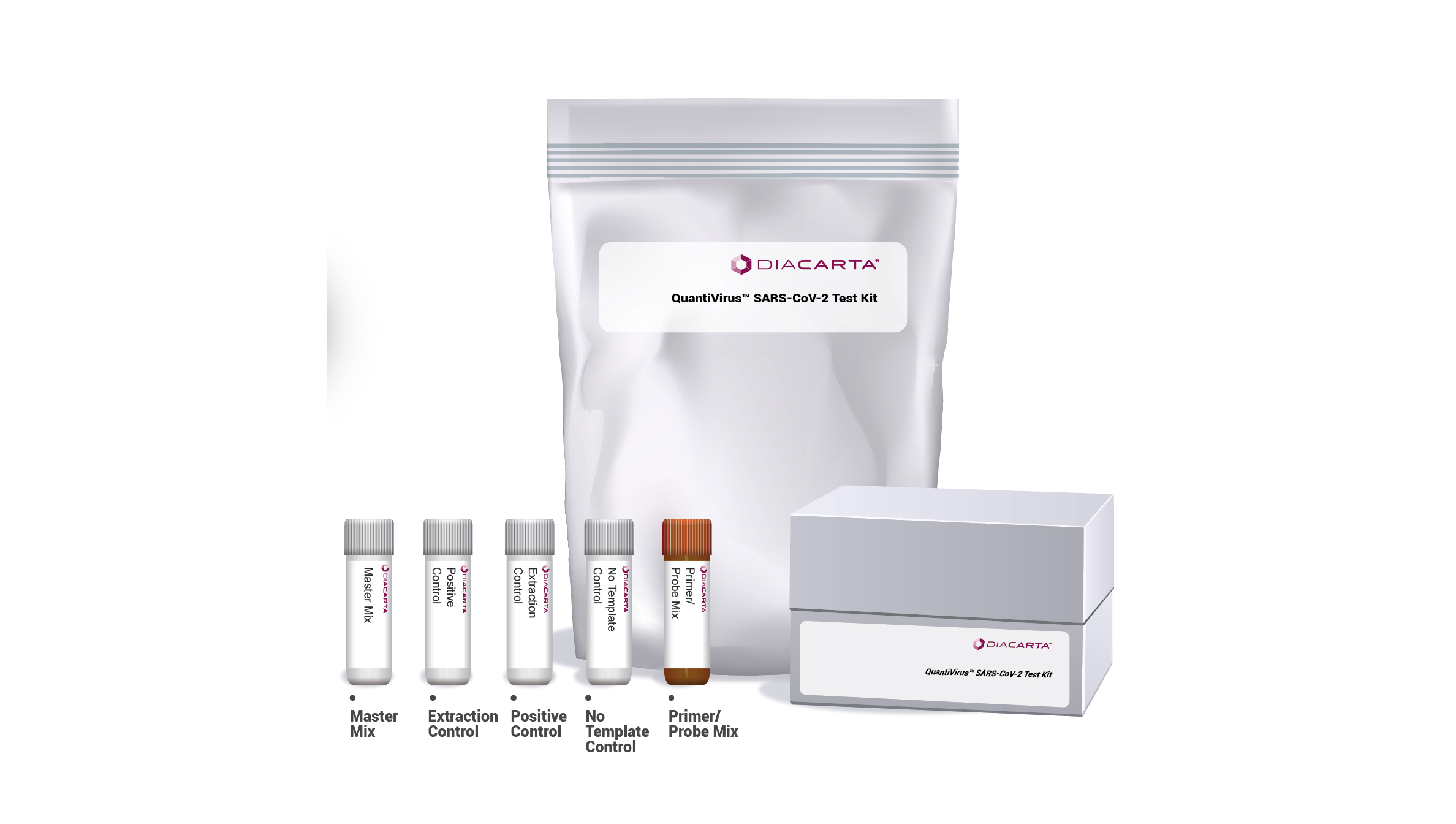
DIACARTA QuantiVirus™ SARS-CoV-2 TEST KIT
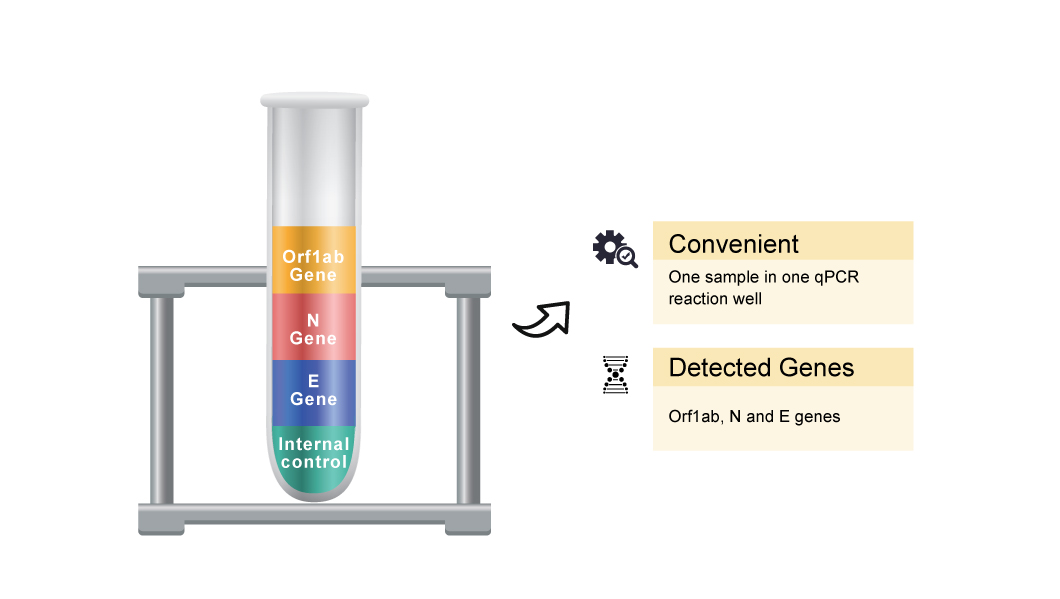
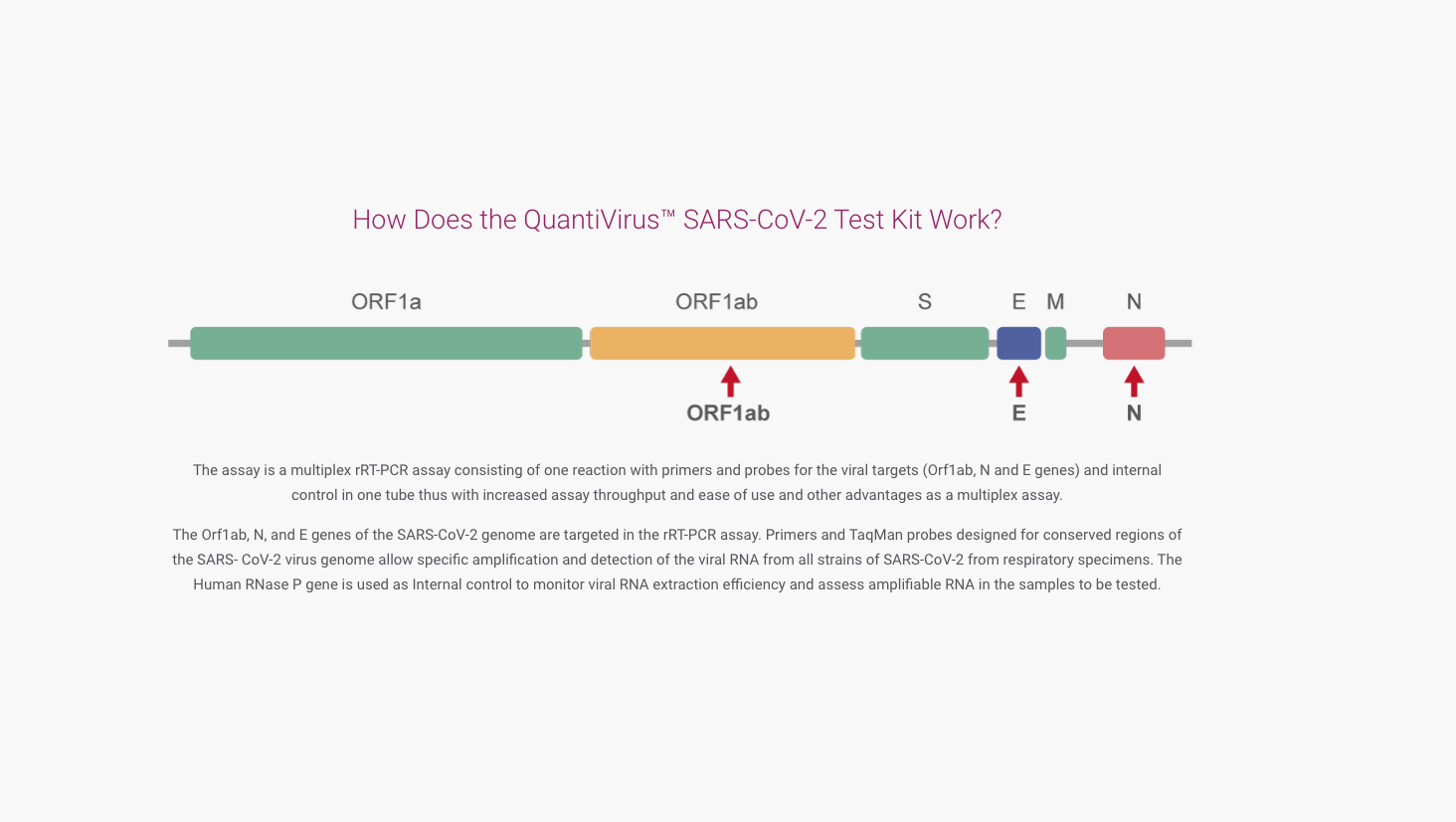
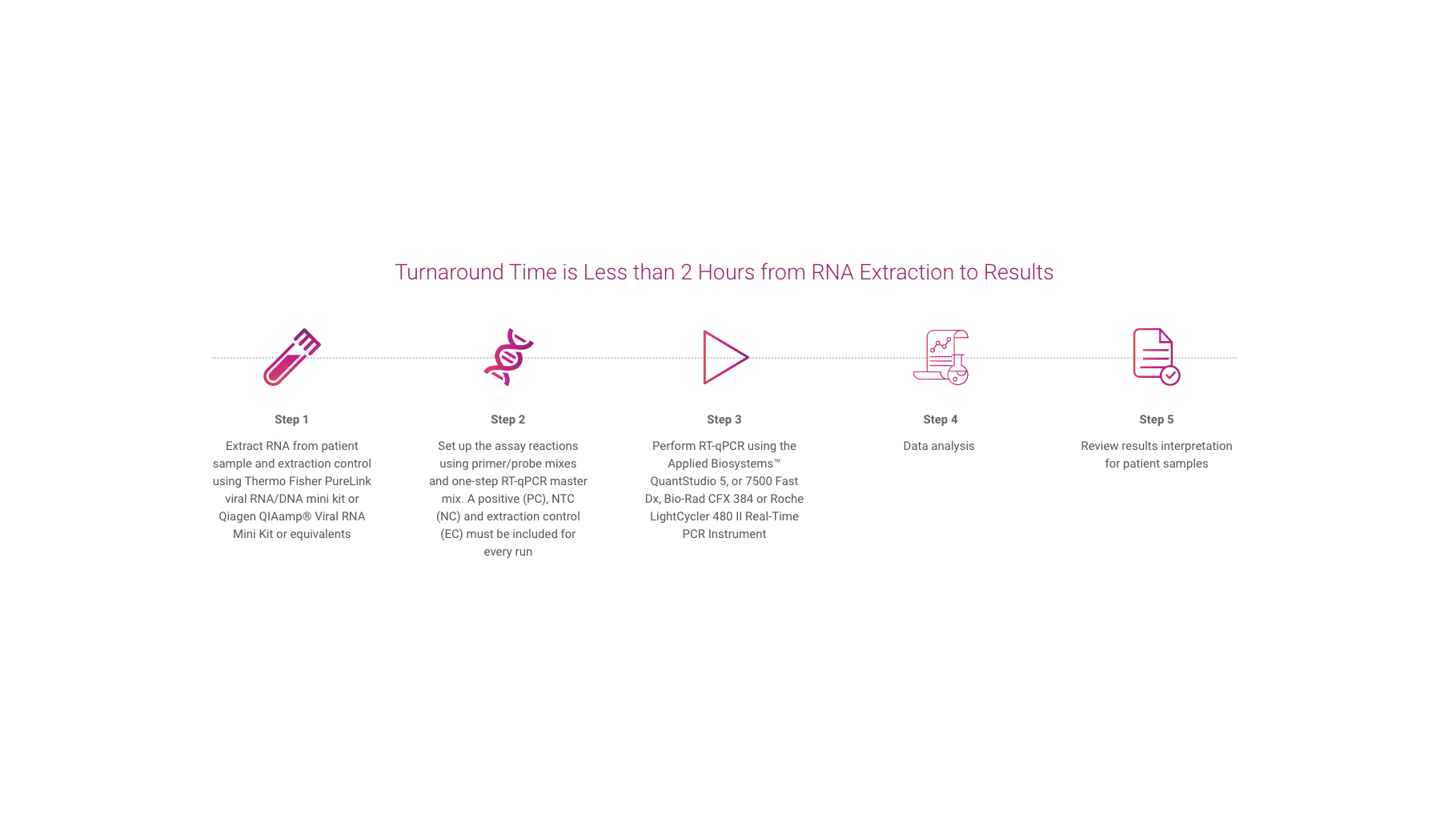
The QuantiVirus™ SARS-CoV-2 Test Kit is based on Real-Time PCR technology, developed for specific detection of SARS-CoV-2 (COVID-19) viral RNA extracted from nasopharyngeal swabs, oropharyngeal swabs and sputum. The analytical sensitivity is 100 copies per mL of SARS-CoV-2 viral with a 95% confidence.
FDA EUA APPROVED I CE/IVD MARKED I MEXICO COFEPRIS APPROVED
INDIA ICRM APPROVED I COLUMBIA INVIMA APPROVED